
DATA MANAGEMENT

The New Era Of Evidence Generation In Clinical Trials
Learn how top pharmaceutical companies are using novel innovations to drive greater speed, scale, and access in clinical research than ever before in this webinar hosted by Musaddiq Khan, Vice President of DCT Solutions at Medable.

Manage Exponential Data Growth With Medidata Clinical Data Studio
Bring study teams together like never before for total data quality oversight.

Streamline EDC Data Entry Using EHR Data
Explore how a clinical research coordinator (CRC) typically manually re-enters data from their EHR/EMR system into Rave EDC, and how Rave Companion provides automated assistance to enable the CRC to complete EDC forms.

The Impact Of AI On Mobile Visits
Experts discuss the FDA's new AI guidance, global harmonization, and the ethical implications of AI in clinical trials. Discover the challenges and opportunities ahead.

ETL: Maximizing Reuse Of Your Clinical Trial Data
ETL — extract, transform, and load — enables sponsors to operationalize clinical trial data, leverage off the investment they have made in other sources to reuse data, and automate populating key elements for registration on CT.gov and EudraCT.

Navigating Trials With Confidence: Building A Robust External Control Arm
Explore methods to build scientifically rigorous comparator groups to allow more patients access to life-saving treatments, accelerate trial timelines, and maximize resources.

Financial Scenario Planning — Budget With Confidence
Find out how financial scenario planning can revolutionize clinical trial budgeting with dynamic models, real-time data, and instant scenario visualization—all in one intuitive platform.

Patient-Level Clinical Trial Data + Real World Data
Future-proof your clinical trial and enhance your evidence generation activities with Medidata Link, which allows sponsors to collect clinical trial data and link it at the patient level to real-world data (RWD).

Verana Health: Where Quality Powers Insights
Drive innovation by improving your digital health in ophthalmology, urology, and neurology.
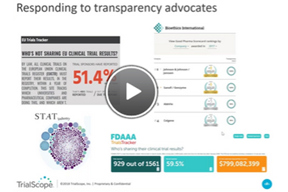
How To Boost Disclosure Efficiencies With Outsourcing
In this webinar, you’ll hear about exciting new advisory and managed disclosure services from TrialScope, including: compliance, policy and process assessments, as well as plain-language summaries, protocol registration, results posting, and redaction services.
...
Here To Help You Succeed
IQVIA Biotech is a standalone business of IQVIA with dedicated teams delivering clinical development solutions for biotech and emerging biopharma customers. Discover why IQVIA Biotech is the CRO partner of choice for biotech companies around the world.

psiXchange: Intelligent, Automated Safety Reporting
Say goodbye to manual burdens and embrace efficiency by fully automating your safety reporting. This will significantly cut down on effort and costs, all while enhancing compliance standards.

Trends In Clinical Transparency Policies
More and more sponsors are making their clinical trial transparency policy publicly available, says TrialScope Chief Strategy Officer Thomas Wicks. Hear additional disclosure and transparency trends.

Amplifying Evidence With Unified Clinical Trial Data Collection
In this presentation, we explore the transformative potential of integrating a unified clinical trial data collection platform with intelligent automation, challenging the inadequacies of the status quo.

Pharmaprojects By Citeline
With Pharmaprojects, leverage your mastery of the R&D space to create winning strategies, identify the right drugs to license, and support the key decisions that will drive your company forward.

The Next Generation Of Clinical Data Management
Discover the challenges that the evolving clinical data landscape brings to clinical data management and what next-generation clinical data management capabilities are needed to address those challenges.

Clinical Data Studio: A Single Data Review Platform Fostering Unification Across Groups
This end-to-end digital review platform can assist both your risk management team and data management team, improving speed and facilitating database lock times.

Helping Sponsors Keep Up With The Latest Clinical Trials Intelligence
Trialtrove helps sponsors form development strategy, anticipate competitive activity, benchmark trial designs and analyze trends. Sponsors also enjoy direct access to industry-leading analysts.

Keeping Up With ClinOps: Why And How To Improve Delivery
Industry experts discuss the concept of a trial platform as a service and how it can accelerate and enhance the day-to-day functions of clinical operations throughout the entire lifecycle of a study.
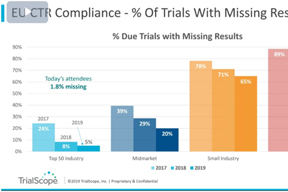
Disclosure & Transparency Trends Webinar
Hear results from TrialScope’s 2019 Global Clinical Trial Disclosure & Transparency Benchmark Survey, highlights from our Plain Language Summaries Survey, and predictions for 2020.

Qdata From Verana Health For HEOR And Medical Affairs
Obtain real-world data on patient demographics, treatment patterns, and outcomes to help inform your future business decisions.

5 Strategies To Dramatically Improve Clinical Data Quality
Discover how to unify data management and risk-based quality management approaches and augment processes with automation and AI to find and fix data quality issues sooner and more efficiently.

The Evolving Role Of AI And Innovation In Cardiac Monitoring
In this presentation, the panel of experts focuses on the changes AI has brought to cardiac monitoring data during clinical trials and addresses the skepticism over the use of new innovations.

Reporting To ClinicalTrials.gov: Reflections And Challenges
Who can reflect better on this topic than Dr. Deborah Zarin, former director of ClinicalTrials.gov? Dr. Zarin, now with the MRCT Center, shared her experiences and advice at the recent EXTRA: TrialScope Transparency Experience.

The Cutting Edge Of Pharma: AI And RWD
Taken from a spotlight session at the CNS Summit, gain insights from thought leaders from Norstella and Citeline regarding artificial intelligence and real-world data.

Beyond The Data Deluge: Immune Profiling In Early Immuno-Oncology Trials
This presentation explores how to design effective immune monitoring for early-phase immuno-oncology trials, focusing on strategic platform selection, cost, and operational feasibility.

Combining De-Identified EHR And Claims Data
Learn how EHR+ Data can improve your understanding of the patient's journey in ophthalmology, urology, and neurology clinical trials.

Castor eConsent: Site View
Study coordinators aim to enroll participants efficiently through a partnership that leverages direct-to-patient marketing to streamline recruitment.

Providing Rich Quality Data In The Neurology Space
From clinical development to post-approval, we provide quality insights powered by the Axon Registry through our exclusive partnership with the American Academy of Neurology.
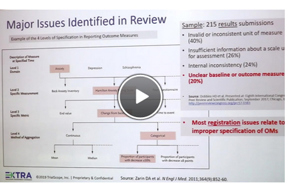
Improving The Quality Of Submitted Studies To ClinicalTrials.gov
Want to reduce QC comments? Hear tips from Dr. Deborah Zarin, former director of ClinicalTrials.gov, now with the MRCT Center. Dr. Zarin was keynote speaker at the recent EXTRA: TrialScope Transparency Experience.
