
TRIAL MANAGEMENT

Clinical Trials And TMF 101 Learning Series: 2nd Session
The “Clinical Trials and TMF 101 Learning Series” kicks off it’s second session with three industry experts, that goes through some of the basics of clinical trials.

What Is The Secret To Ongoing TMF Health?
Delve into specific tactics clinical teams can utilize for establishing immediate and long-lasting improvements to their TMF business processes.
Defining And Implementing The Right Oncology Digital Strategy
A panel of oncology experts shares how top life science companies are using a combination of DHTs, ePROs, clinical trial platforms, and more to ease oncology research and drastically reduce timelines.

Navigating Early Phase CNS-Active Drug Development
Early development of CNS-active drugs requires in-depth clinical expertise. Altasciences’ Dr. Beatrice Setnik, Chief Scientific Officer, examines the strategies to ensure early comprehensive data collection to fulfill regulatory requirements and aid in evaluating th...

At The Crossroads Of Data: eCOA And Sensors Converging For Novel Insights
Leveraging sensors and eCOA addresses increasing clinical trial complexity and ensures a more efficient and effective study design.

Perceptive eClinical
Learn about a randomization and trial supply management (RTSM) solution that helps keep your trial on track.
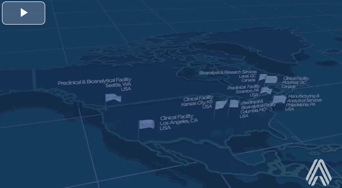
Altasciences Has You Covered From Coast To Coast
With your success in mind, we designed an integrated early phase drug development offering with eight locations, to get you from lead candidate selection to clinical proof of concept, and beyond.

Applying Virtual Approaches To Dermatology Trials
Explore how to integrate virtual approaches into the design and delivery of dermatology trials – to mitigate study risks and enhance patient engagement.
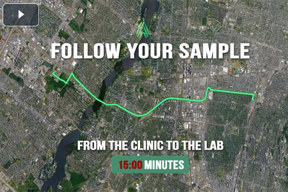
Accelerate Your Data With Our Co-Located Clinic And Lab
Watch as we take your fresh sample from our clinic to PBMC separation. This video overviews our optimized workflows that ensure timely processing, transport, and analysis of your samples.
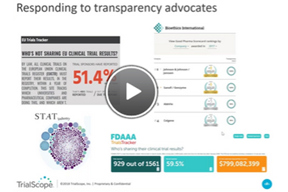
How To Boost Disclosure Efficiencies With Outsourcing
In this webinar, you’ll hear about exciting new advisory and managed disclosure services from TrialScope, including: compliance, policy and process assessments, as well as plain-language summaries, protocol registration, results posting, and redaction services.
...
Learning About The Sensors In myMedidata
Learn how sensor data and advanced analytics can transform clinical research and enable your study to overcome data challenges, improving patient care.

Strategic Considerations For A Successful CNS Clinical Development Pathway
Dr. Beatrice Setnik, Chief Scientific Officer at Altasciences, discusses tactics for a successful clinical development pathway for CNS-active drugs.

Keeping Up With ClinOps: Why And How To Improve Delivery
Industry experts discuss the concept of a trial platform as a service and how it can accelerate and enhance the day-to-day functions of clinical operations throughout the entire lifecycle of a study.

Practical Approaches To Faster Study Start-Ups
Learn how teams can leverage organizational, procedural, and technological solutions to recapture valuable days, weeks, and even months in the activation timelines of research sites.

Driving Simulation Studies At Altasciences
At Altasciences, we design driving studies to achieve your objectives, leveraging extensive experience across clinical testing of cognitive effects.

The Golden Era Of GLP-1 Drugs
As GLP-1 drugs reshape the market, understanding their potential risks, applications, and economic effects is crucial for professionals in the pharmaceutical and healthcare industries.

Do Mobile Visits Drive Faster Study Results?
Discover solutions to clinical trial challenges — this on-demand webinar by PCM Trials and Clinical Leader offers insights into the role of mobile nurse visits in overcoming patient dropout and trial delays.

The Path Forward, Altasciences' Approach To Project Management
Learn about a unique, integrated program management strategy that can lower your costs and reduce program timelines from lead candidate selection to clinical proof-of-concept by up to 40%.

Solving The Impossible In Glioblastoma With An AI Synthetic Control Arm
This video discusses Medicenna’s MDNA55-05 clinical trial; a single-arm, open-label, multicenter study that offers a promising opportunity to improve outcomes for recurrent glioblastoma.

Modernizing Clinical Research With Patient-Centric Digital Data Collection
Accelerate clinical trial modernization with streamlined collection of continuous, high-precision data centered on the patient.

TMF Management In Clinical Trials
Elevate your knowledge of clinical trials and the Trial Master File by exploring three critical topics.

Think U.S.A.: The Operations Behind Your Clinical Trials
Ready to think differently about early-phase clinical development? Check out the spotlight on our U.S. clinical facilities and the incredible teams behind them.

Are Your Clinical Study Meetings Building Momentum, Or Wasting Time?
Transforming passive clinical trial meetings into active catalysts for success, aligning teams and accelerating timelines.

Clinical Research Without Borders
Explore strategies for navigating global clinical trials with, Afshawn Chakamian of Avidity Biosciences, Dr. Amy Raymond of Worldwide Clinical Trials, Scott Schliebner of Novotech, and Scout’s KimberLee Heidmann.

Patient First Solutions For Oncology
Medable, Inc's new oncology offering is an end-to-end suite that includes an extensive eCOA library, pre-built and validated DCT applications such as Total Consent and Televisit, and protocol design consulting.

Optimize Protocols For More Successful Trials
Find out how you can harness the power of AI and world-class data to recommend endpoints and inclusion/exclusion criteria for streamlined protocol design that results in more positive trial outcomes.

Build Your Diversity Action Plan With AI And Patient Insights
Discover how AI and patient insights can help you build a compliant Diversity Action Plan and overcome barriers to achieve diverse clinical trial enrollment.
Webinar: Reimbursement Methods For Sustainable CGT Business Models
Learn more about the key features of successful innovative pricing and reimbursement models as well as outcomes-based agreements and the ways to advance those strategies for cell and gene therapies.

Tackling The EU CTR Compliance Challenge
Maintaining disclosure compliance is tricky enough with ClinicalTrials.gov, but when it comes to EU CTR compliance, the complexity rises to a whole new level. As a sponsor, do you find your organization “guilty” of these common errors?

Investigator SmartSelect
This advanced recommendation engine identifies protocol-specific investigators in just minutes, dramatically shortening a process that typically takes four to six weeks.
